Background
HHV-8-negative/Idiopathic Multicentric Castleman Disease (iMCD) is a rare lymphoproliferative disorder with limited treatment options and poor outcomes. Siltuximab remains the only FDA approved treatment for the disease (April 2014), but its uptake and associated outcomes outside of clinical trials remain unclear. Further, constructing a large enough cohort of patients with iMCD to better understand siltuximab use in real-world practice is a challenge due to its low incidence, lack of a Castleman Disease (CD)-specific ICD code until 2017, and effort required for manual chart abstraction. We built a natural language processing-based machine learning (ML) model to select patients with iMCD from a nationwide electronic health record (EHR)-derived database and described their clinical characteristics, treatment trends, and real-world overall survival (rwOS).
Methods
Across a cohort of over 3.5 million patients in the EHR-derived, Flatiron Health database (which comprises de-identified patient-level structured and unstructured data from ~280 US cancer clinics and ~800 sites of care), patients with ML-extracted diagnoses and dates of diagnoses for iMCD and a visit on or after 2011 were selected. Precision (positive predictive value) of the ML-selected cohort was measured on 50 human abstracted charts. Descriptive statistics summarized patient characteristics and treatment trends. Treatment patterns were assessed among patients diagnosed on or before January 31, 2023 to allow a minimum of 3 months for treatment initiation. Changes over time in first-line (1L) treatment status were measured by diagnosis year category (<2014, 2014-2018, 2019-2023), and were chosen based on siltuximab approval date (2014) and publication of treatment guidelines for iMCD (2018). 1L regimens were grouped into one of the following mutually exclusive categories in hierarchical order: siltuximab-containing regimen, rituximab-containing regimen, and primary chemotherapy-containing regimen. 5-year rwOS rates for the entire iMCD cohort (indexed to diagnosis date), as well as patients with 1L-siltuximab or non-siltuximab regimens (indexed to treatment start date) were estimated using the Kaplan-Meier estimator. Patients with a diagnosis date before 2011 were excluded from survival analyses to avoid immortal time bias.
Results
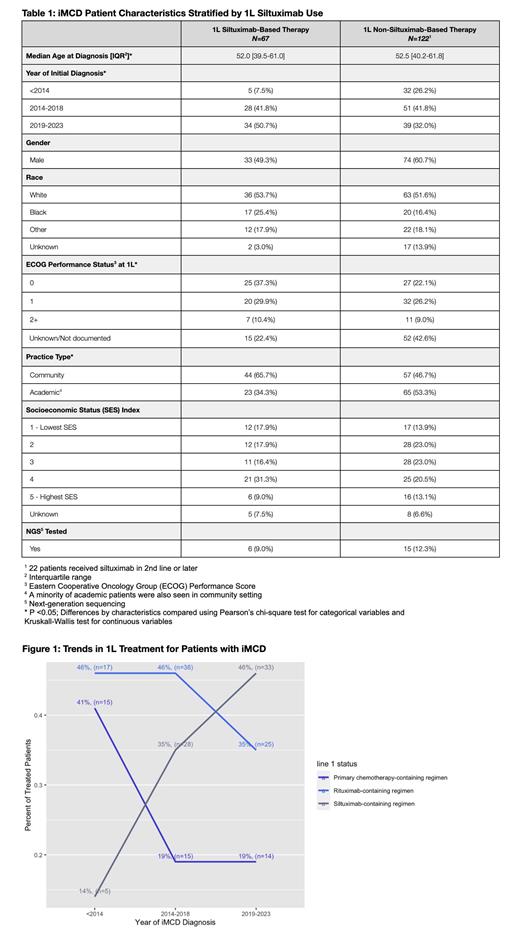
The ML model selected 267 patients as having iMCD across the Flatiron network. The precision of the model was 90% for a CD diagnosis (including unicentric, HHV-8-associated multicentric, or unclassified CD) and 76% for the iMCD cohort. Patients with iMCD were predominantly male (55%), white (53%), and had a median age at diagnosis of 52. Compared to patients treated with non-siltuximab 1L therapy (N=122), patients treated with siltuximab-based regimens in 1L (N=67) were more likely to have been diagnosed more recently, seen in the community setting, and have a documented ECOG performance status (p<0.05) but were otherwise similar (Table 1). Among patients with evidence of treatment, use of 1L siltuximab-based regimens increased from 35% of patients diagnosed 2014-2018 to 46% diagnosed 2019-2023 while 1L rituximab-based regimen use dropped from 46% to 35%. 1L primary chemotherapy-based regimen use dropped from 41% prior to 2014 to 19% post 2014 (Figure 1). Among patients diagnosed after 2014 and who had evidence of treatment, 51% (77/151) received siltuximab at any point. Unadjusted 5-year rwOS rate for the entire iMCD cohort, including patients without evidence of treatment, was 84% [95% CI: 78-90%]. For patients treated with a 1L siltuximab-containing regimen, unadjusted 5-year rwOS was 86% [95% CI: 78%-96%], whereas for patients treated with non-siltuximab based 1L therapy, unadjusted 5-year rwOS was 81% [95% CI: 72%-90%].
Conclusion
ML extraction allowed for the largest analysis of real-world patients with iMCD to date. While use of siltuximab in the 1L setting is increasing over time, only half of patients with evidence of treatment receive it at any point. Unadjusted 5-year rwOS rates for those treated with both siltuximab and non-siltuximab based 1L therapy was higher than previously reported. This may be due to the inclusion of other CD subtypes in the cohort or sicker patients not being seen in an outpatient oncology setting. Future work should explore the drivers of poor outcomes for patients as well as the factors associated with lack of receipt of siltuximab.
OffLabel Disclosure:
Cohen:Flatiron Health, an independent subsidiary of the Roche Group: Current Employment, Current equity holder in publicly-traded company. Estevez:Flatiron Health, an independent subsidiary of the Roche Group: Current Employment, Current equity holder in publicly-traded company. Kelly:Flatiron Health, an independent subsidiary of the Roche Group: Current Employment, Current equity holder in publicly-traded company. Gippetti:Flatiron Health, an independent subsidiary of the Roche Group: Current Employment, Current equity holder in publicly-traded company. Fidyk:Flatiron Health, an independent subsidiary of the Roche Group: Current Employment, Current equity holder in publicly-traded company. Brandstadter:EUSA Pharma: Consultancy. Fajgenbaum:EUSA Pharma/Recordati Rare Disease: Consultancy, Research Funding; Medidata, a Dassault Systemes company: Consultancy.
Idiopathic Multicentric Castleman Disease (iMCD) has only one FDA approved therapy (siltuximab). Rituximab is frequently used off-label for the treatment of iMCD and is a recommended treatment in the International Consensus iMCD treatment guidelines (https://doi.org/10.1182/blood-2018-07-862334)